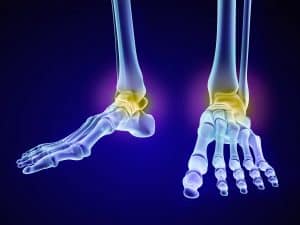
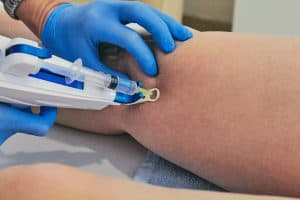
Stem Cell Therapy Pittsburgh PA for Foot & Ankle Pain


Stem Cell Therapy
Regenerative Medicine in podiatry:
using non-surgical stem cell therapy and platelet treatments to avoid surgery of the foot and ankle.
Surgery should be an option of last resort, a powerful tool used only when all less-invasive treatments have failed. Suffice it to say that modern, technology-driven surgical procedures are incredibly sophisticated and useful. Yet they will always carry some risk of unwanted “side effects” and other complications. It ultimately doesn’t matter how minimally invasive a procedure is. The skin is being broken and body tissue is being physically altered, often permanently. Avoid surgery with Stem Cell Therapy.
So even while surgery as a whole has become safer, easier, and more effective medical professionals have constantly pushed for ways to avoid it. This has led to the development of entirely new branches and specialties, including regenerative medicine. Right now, thanks to these procedures, we’re finally becoming able to coax tissue to heal itself. This avoids the risks and downsides of surgery entirely.
Common Foot and Ankle Problems
This is especially true for relatively common foot and ankle injuries where complete healing and regeneration is what’s truly necessary. In many surgical cases all that’s being accomplished is a “clean up” job of damaged cartilage or other structures. The physical stitching together, shortening, and tightening of tendons and ligaments is also, unfortunately, routine. To date, outside of transplant surgery, there are no procedures which can add healthy, undamaged tissue to an existing injury.
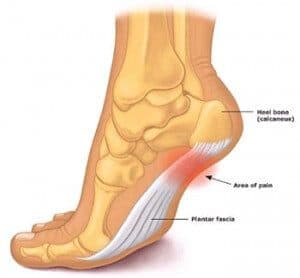
Stem Cell Therapy for Plantar Fasciitis
Plantar fasciitis surgery is an outstanding example. The plantar fascia is the long, thick, extremely tough tendon which occupies the bottom of the foot. Due to its location, this structure withstands an enormous amount of stress and strain with each and every step. Over time, this leads to the accumulation of small amounts of damage until a tipping point is reached.
When a patient begins to feel the often intense pain of plantar fasciitis it’s too late, as the damage has been done. While surgery can accomplish some of “off-loading” or “decompression” of this tendon it can’t undo the underlying trauma. Fortunately, regenerative therapies can in many cases.
Stem Cell Therapy – better than steroid injections!
Both of these treatments can also achieve the same goals as steroid injections, e.g. making painful symptoms disappear. Regenerative treatments share none of the potentially severe side effects of medications like Cortisone, however. The results obtained typically last much longer—once the tissue is healed the relief can last indefinitely.
In many ways, steroid injections and treatments like stem cell therapy produce exactly the opposite effects. Steroids resolve symptoms by eliminating all inflammation. While effective, this can greatly impede the healing process, gradually making it worse. Conversely, stem cell therapy resolves symptoms by triggering the healing process. As the tissue regenerates symptoms naturally lessen and may disappear completely.
It’s safe to say that steroids are extremely useful medications that will always have a place in orthopedic medicine. They should be used sparingly and with caution however, especially in chronic injuries when incomplete healing is suspected.
Considering surgery for your plantar fasciitis pain? Read this first.
One of the most attractive aspects of regenerative medicine is its ability to delay or eliminate the need for surgery. This makes treatments such as stem cell therapy, Platelet Rich Plasma (PRP) injections, and related techniques uniquely suited to the treatment of plantar fasciitis.
Stem cell therapy is a completely natural, injection based treatment which is designed to stimulate the body’s regenerative abilities. Prior to treatment, a patient’s own living stem cells are harvested and concentrated into an injectable serum. In many cases other blood cells or cell-like particles, i.e. platelets, are included into the mix. Once introduced into damaged tissue a powerful, localized healing response results. In this way chronically damaged structures can be made to heal properly and completely.
Below is a brief overview of plantar fasciitis. Please not that regenerative therapies can be effective on a wide variety of other long-standing soft tissue injuries as well.
The plantar fascia:
A thick, exceedingly tough band-like tendon which runs the length of the plantar surface (bottom) of the foot. It has a web-like appearance and connects the toes and forefoot to the anterior surface (front) of the heel.
The plantar fascia plays a vital role in supporting the arch of the foot. Any condition which affects this tendon can have a negative impact on a patient’s gait and mobility. Patients with advanced cases often present with difficulty walking even short distances due to excessive pain.
Choosing a stem cell Dr for Your Ankle and Plantar Fascia:
Do they have a sole focus on ankle Regenerative Medicine?
Many different doctors would like you to believe that they offer true ankle Regenerative Medicine. In reality, the treatments they give range from first-quality to completely ineffective. It’s much more likely that you’ll receive a lesser-quality treatment from non-foot and ankle specialists, however.
Lastly, some Chiropractic clinics, Acupuncture and Oriental Medicine (AOM) clinics are claiming to offer Regenerative Medicine. Put simply, they don’t. Most are just using technical-sounding medical language to promote questionable treatments. These practitioners are not DPMs, and they can’t (and certainly shouldn’t) perform treatments such as ankle/ plantar fascia Prolotherapy or PRP.
What are the potential risks and side effects of stem cell therapy?
Stem cell therapy holds promise for treating a range of medical conditions, but like any medical intervention, it also comes with potential risks and side effects that need to be carefully considered.
One major concern is the risk of uncontrolled cell growth, leading to the development of tumors or cancers. When stem cells are introduced into the body, there’s a possibility that they could differentiate into unintended cell types, causing abnormalities. This risk is particularly associated with embryonic stem cells and induced pluripotent stem cells (iPSCs), as they possess a higher capacity for uncontrolled growth.Immune rejection is another issue. If stem cells are derived from a donor source, the recipient’s immune system might recognize them as foreign and launch an immune response, potentially leading to graft-versus-host disease (GVHD) or rejection of the transplanted cells.
Additionally, there’s a risk of infection during the collection, processing, or transplantation of stem cells. If proper sterilization and quality control measures aren’t followed, infections could occur, posing a threat to the patient’s health.
Ethical concerns also loom large, particularly with the use of embryonic stem cells. The destruction of human embryos for research purposes raises ethical questions and debates.
While many clinical trials have demonstrated the safety of stem cell therapies, the long-term effects are not always fully understood. Some side effects might not surface until years after the treatment. Regulatory concerns also play a role. The availability of unregulated stem cell treatments in some countries has led to cases of patients receiving ineffective or even harmful therapies.
It’s essential for patients and medical professionals to be aware of these potential risks and side effects. Rigorous research, stringent regulation, and well-designed clinical trials are crucial to ensure the safety and efficacy of stem cell therapies.
Do they use Prolotherapy? If so, how???
Prolotherapy is the oldest form of Regenerative Medicine, and some practitioners think of it as the most effective. It’s the original minimally-invasive joint injection treatment that ultimately led to the treatments mentioned above.
Can the doctor you’re considering perform Prolotherapy? Also, if the answer is yes, how are they performing it?
Many newer clinics offer prolotherapy treatments that are not sufficient to get the best results. These doctors are indeed providing Prolotherapy, but may give as little as 3 injections. Another doctor at a dedicated Regenerative Medicine clinic may have injected the same joint 30 times or more. One is treating the whole joint while the other isn’t, and there will almost certainly be huge differences in patient improvement.
Prolotherapy is regarded as one of the most technically difficult methods used in Regenerative Medicine. If a potential doctor makes 15-20 injections to treat a chronically sprained ankle, then they’re probably a good selection.